Corneal Light Eye Assessment
Introducing the CLEA device: Redefining Corneal Imaging Through Precision, Portability, and Simplicity. At the forefront of ocular diagnostics, CLEA represents a breakthrough in corneal imaging—combining clinical-grade accuracy with unmatched mobility. Engineered for the modern clinician, CLEA transforms the traditional topography workflow by offering a lightweight, smartphone form-factor imaging platform that delivers high-resolution corneal scans anytime, anywhere.
Innovation at the core of CLEA
CLEA’s innovation lies in its seamless integration and rapid analysis of thousands of data points on the corneal surface, empowering practitioners with granular, highly accurate maps of the corneal topography—comparable to leading desktop systems. With an accuracy of ±0.07 D and algorithmic intelligence capable of detecting a wide range of corneal pathologies, CLEA offers a robust alternative to conventional bulky instruments.

No barriers
Whether you're in a clinic, satellite office, or on the move, CLEA ensures clinical confidence without compromise. Its FDA-exempt Class I status allows licensed healthcare professionals to use the device without regulatory barriers, streamlining workflows and expanding accessibility in primary eye care and specialty practices alike.
Technology to scale at speed
CLEA's imaging application is optimized and built with modularity to enable:
-
Edge computing for real-time rendering
-
AI/ML-enhanced diagnostic algorithms for disease progression analysis
-
EHR and DICOM/FHIR integration (roadmap)
-
HIPPA Compliance
-
Blockchain-ready architecture for secure audit trails and patient data protection
Mobility Without Compromise
CLEA’s compact form factor and rapid scan capability provide unprecedented flexibility in corneal imaging—extending diagnostic reach into domiciliary care, rural clinics, and satellite sites. Software upgrades delivered over the cloud and modular design ensure that you are always up to date with the state-of-the-art technology.
The CLEA Process
CLEA’s advanced imaging technology and algorithms track changes in corneal health over time, helping identify potential vision impairments before they progress to advanced stages.

Stage 1:
Auto-capture rapidly acquires a high-resolution image of the cornea and anterior segment.

Stage 2:
Digital analysis of over 7000 data points generates quantitative and qualitative topographic images of the corneal surface.
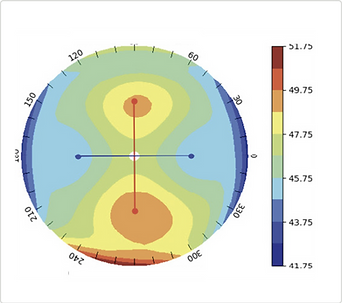
Stage 3:
Algorithmic reconstruction of the corneal surface generates quantitative keratometry results visualized on a corneal topography colormap for the optometrist.

Stage 4:
Final report in PDF format shared directly from the CLEA app or patient portal with the patient and medical / healthcare professional.
Milestones
Conceptualization
2021
Product ideation and design evolution.
Incubation and
IP Registration
2021 - 2024
Product incubation for a 3-year period, concluding with the first, FDA validated and production ready CLEA device.
FDA Exemption
2024
The CLEA device achieved FDA exemption in 2024.
In-Market Trials
2024 - Now
Vision Labs succesfully introduced CLEA into partner hospitals and practices.
Who Benefits
Patients
The cornea is responsible for more than 70% of vision clarity, but only 5% of patients have corneal screening as part of their eye exam, CLEA has the potential to ensure a more comprehensive eye exam is feasible due to its simplicity. In addition, the patient gets a full copy of their screening result, allowing for improved monitoring over time.
Optometrists
A corneal topographer is viewed as specialist equipment, so CLEA provides an Optometrist the ability to offer improved screening. This in turns enables an Optometrist to be more accurate in screening for certain conditions and be more specific in referring patients for specialist care, improving the downstream impact on specialist resources, while improving patient care.
Ophthalmologists
In many states the patient waiting time to see a specialist ranges between 3 to 6 months. Improved upstream screening of patients reduces the load of screening on Ophthalmologists, enabling these specialists to focus on treatment of confirmed conditions. This will not only improve the waiting time for patients to see a specialist, but it will also improve the overall utilisation of specialist skills and care in general.
Healthcare Professionals
CLEA is certified for use by healthcare professionals, opening interesting opportunities for extended or mass screening initiatives, both in the US market, such as school programs, and emerging markets, where it is typically led by NGOs.

